Multiple myeloma (MM) is a hematological malignancy which characterized by malignant plasma cells resident in the bone marrow (BM). The BM microenvironment plays critical roles in the invasion, proliferation and migration of myeloma cells. Despite the therapies of MM improved in recent years, this disease is still incurable and requires precise treatment. Resident memory (TRM) cells are a distinct tissue-localized T cell lineage that is crucial for protective immunity in peripheral tissues. Recent studies reveal TRM cells as a vital component of the host immune response to cancer. TRM-like tumor-infiltrating lymphocytes (TILs) can be found in a wide range of human cancers, where they portend improved prognosis. However, the precise role and mechanism of bone marrow TRM cells in MM still remain elusive.
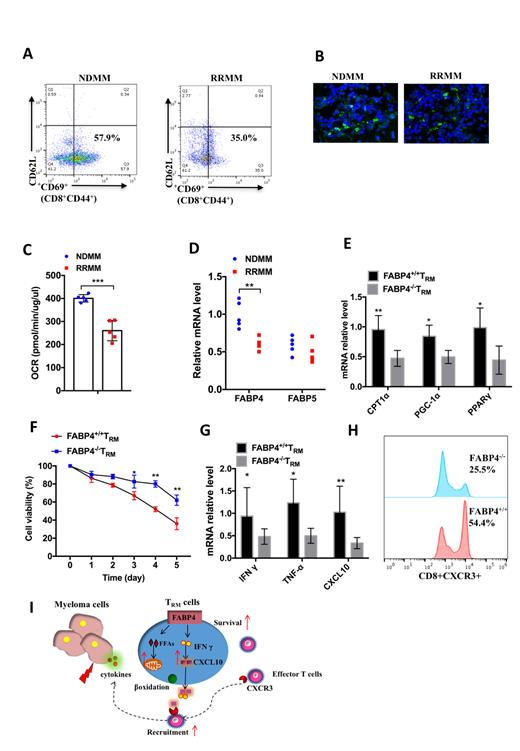
In present study we first found that the accumulation of T RM cells (CD8 +CD44 +CD69 +CD62L -) in BM newly diagnosed MM (NDMM) patients was significantly higher than that of relapse recurrent MM (RRMM) ( Figure A), which was further confirmed by the immunohistochemistry (IHC) staining analysis ( Figure B). By using Seahorse XF e analyzer we found a decrease of oxygen consumption rate (OCR) of T RM cells in RRMM comparing to that of NDMM ( Figure C). Since fatty acid metabolism is important in β oxidation, we further investigated the expression of fatty acid binding proteins (FABP4/FABP5) in T RM cells and showed that FABP4 was dominant in T RM cells of NDMM patients ( Figure D). FABP4 +/+ mice and their relative controls FABP4 -/- mice were adopted to further explore the role and mechanism of T RM cells in MM progression. The abundance of genes related fatty acids β oxidation was significantly decreased in FABP4 -/- T RM cells ( Figure E). The tumor cell viability was decreased in myeloma cells co-cultured with FABP4 -/- T RM cells in the presence of bortezomib ( Figure F). Moreover, the expression of cytotoxic cytokines including IFN γ, TNFα and CXCL10 was significantly higher in FABP4 -/- T RM cells ( Figure G). The alarming function of T RM cells was further enhanced via recruiting more effector T (CD8 +CXCR3 +) cells ( Figure H), therefore the apoptosis of tumor cells was elevated in BM of MM patients.
In summary, T RM cells are necessary and sufficient for long-lived protection against tumors. T RM cells derived FABP4 exerts anti-tumor immunity through prolonging the survival of T RM cells and enhancing the recruitment of more effector T cells to suppress the tumor progression ( Figure I). This study suggests that T RM cells might serve as a novel therapeutic target in MM and treatment specific targeting FABP4 might shed light on the development of potential therapeutic strategies for treating MM especially relapse patients.
Acknowledgement
This project is supported by the Sun Yat-sen University Hundred Talents Program (PT19200101), Natural Science Foundation of Guangdong Province (2022A1515010290).
Disclosures
No relevant conflicts of interest to declare.